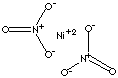|
PHOTOCHEM Nickel Nitrate crystals per 100gm (05951-0100)
MSDS Number: N3030 * * * * * Effective Date: 11/21/08 * * * * * Supercedes: 01/09/06
NICKEL NITRATE1. Product IdentificationSynonyms: Nickel (II) nitrate, hexahydrate (1:2:6); nickelous nitrate; nitric acid, nickel (2+) salt, hexahydrate; Nickelous nitrate, 6- Hydrate 2. Composition/Information on Ingredients
Ingredient CAS No Percent Hazardous --------------------------------------- ------------ ------------ --------- Nickel Nitrate 13138-45-9 90 - 100% Yes 3. Hazards IdentificationEmergency Overview 4. First Aid MeasuresInhalation: 5. Fire Fighting MeasuresFire: 6. Accidental Release MeasuresRemove all sources of ignition. Ventilate area of leak or spill. Wear appropriate personal protective equipment as specified in Section 8. Spills: Clean up spills in a manner that does not disperse dust into the air. Use non-sparking tools and equipment. Reduce airborne dust and prevent scattering by moistening with water. Pick up spill for recovery or disposal and place in a closed container. 7. Handling and StorageKeep in a tightly closed container, stored in a cool, dry, ventilated area. Protect against physical damage and moisture. Isolate from any source of heat or ignition. Avoid storage on wood floors. Separate from incompatibles, combustibles, organic or other readily oxidizable materials. Areas in which exposure to nickel metal or soluble nickel compounds may occur should be identified by signs or appropriate means, and access to the area should be limited to authorized persons. Containers of this material may be hazardous when empty since they retain product residues (dust, solids); observe all warnings and precautions listed for the product. 8. Exposure Controls/Personal ProtectionAirborne Exposure Limits: 9. Physical and Chemical PropertiesAppearance: 10. Stability and ReactivityStability: 11. Toxicological Information
--------\Cancer Lists\------------------------------------------------------
---NTP Carcinogen---
Ingredient Known Anticipated IARC Category
------------------------------------ ----- ----------- -------------
Nickel Nitrate (13138-45-9) Yes No 1
12. Ecological InformationEnvironmental Fate: 13. Disposal ConsiderationsWhatever cannot be saved for recovery or recycling should be handled as hazardous waste and sent to a RCRA approved waste facility. Processing, use or contamination of this product may change the waste management options. State and local disposal regulations may differ from federal disposal regulations. Dispose of container and unused contents in accordance with federal, state and local requirements. 14. Transport InformationDomestic (Land, D.O.T.) 15. Regulatory Information --------\Chemical Inventory Status - Part 1\---------------------------------
Ingredient TSCA EC Japan Australia
----------------------------------------------- ---- --- ----- ---------
Nickel Nitrate (13138-45-9) Yes Yes Yes Yes
--------\Chemical Inventory Status - Part 2\---------------------------------
--Canada--
Ingredient Korea DSL NDSL Phil.
----------------------------------------------- ----- --- ---- -----
Nickel Nitrate (13138-45-9) Yes Yes No Yes
--------\Federal, State & International Regulations - Part 1\----------------
-SARA 302- ------SARA 313------
Ingredient RQ TPQ List Chemical Catg.
----------------------------------------- --- ----- ---- --------------
Nickel Nitrate (13138-45-9) No No No Nickel cmpd/
--------\Federal, State & International Regulations - Part 2\----------------
-RCRA- -TSCA-
Ingredient CERCLA 261.33 8(d)
----------------------------------------- ------ ------ ------
Nickel Nitrate (13138-45-9) No No No
Chemical Weapons Convention: No TSCA 12(b): No CDTA: No
SARA 311/312: Acute: Yes Chronic: Yes Fire: No Pressure: No
Reactivity: Yes (Pure / Solid)
WARNING: 16. Other InformationNFPA Ratings: Health: 1 Flammability: 0 Reactivity: 0 Other: Oxidizer No accessories for this product. |
  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ECommerce Integration by Yart
(Packing, handeling and shipping charges have been incorporated in the cost of these goods)
Today we are offering free freight in Australia on this product.Small items will ship via Australia post. All other products will ship via road freight or local couriers.This is a limited offer and subject to change at any time.
For Lab service products the return post or freight has been incorporated in the price and for Drop box only services there is NO freight applicable.
Close